
What if instead of using the usual methods to create chemicals, fuels and other products, we can employ life itself, harnessing the double helix to power our industry?
Using living microorganisms to carry out industrial functions has existed on a relatively small scale for some time. However, it has been difficult to scale up engineered — or synthetic — biology to an industrial scale, and thus making the processes commercially viable has remained out of reach. That is now changing.
The biggest hurdle in getting microbes to do your bidding at a large scale is the same thing that makes them so interesting in the first place – they’re living things, and they respond to their environment as living things do.
They’re not always predictable, they’re sensitive to where they live, produce waste by-products that are useless to the process, and – like all living things – they die. Individual organisms can malfunction just as easily as man-made tech. For microbes, whose lifespans can sometimes be measured in days, evolution itself becomes a force that needs to be factored in.
A big part of the solution is genetic muting: silencing the parts of an organism’s genetic code that don’t serve you. You stop them from expressing any elements or functions other than exactly those which perform the task you want them to.
Third wave
The road to engineering biology for industry can be roughly broken up into three waves. The first wave began with figuring out how to use plants or organic fermentation systems to produce desired outputs in the first place. It was followed by a second wave, which sought to leverage emerging technologies like AI and automation to speed up the process and reduce the cost of the process design.
The third wave, which we have now entered, takes a deeper dive inside the cell itself and engineers it to do exactly what you want, reducing the cost of the actual end-product.
One area that may benefit from this third wave is biofuel — a potential key to decarbonising the transport sector, particularly areas like aviation, but challenging to produce at scale. However, companies like Viridos, which is using algae to produce biofuels for heavy transport like aviation, are demonstrating new ways to ramp up production.
What Viridos has been able to successfully demonstrate where most others have not, is the ability to take these algal populations and cultivate them outdoors – essentially in big ponds – as opposed to the controlled indoor tanks that may be more efficient but are far costlier and more difficult to scale. The challenge is controlling the population so that it can survive any contamination in the pond, but also not growing so fast it becomes toxic to itself.
Last summer, Viridos hit a production milestone of 9.1 grams of bio-oil per metric metre per year for its open pond system – 10 grams is considered the threshold beyond which the system becomes economically viable.
Another sector making use of synthetic biology is food – both for humans and livestock. Companies like California-based Calysta are working to make animal feed/food material using gases as an input.
Gases, including nitrogen and oxygen, are used as feedstock for microbes, which then produce protein that gets collected and reconfigured into different kinds of feed. It is starting with feed for fish and other livestock, with plans to expand into pet food markets and ultimately into ingredients for human food.
Breaking into the human food supply chain will be more of a challenge, as microbe-based protein may sound unappetising to the ear of the average consumer. One thing it does have going for it is the contrast with other proteins like insects, which trigger a stronger disgust response from consumers. The popularity of other microbe-based products such as spirulina and miso also bode well.
The chemicals sector, like so many others, is now looking to decouple from fossil fuels.
Using gas as feedstock also sets up a convenient symbiotic relationship with energy companies, which often have to deal with the costly problem of stranded assets – having to store gas somewhere without an economically viable way to transport it to market. This kind of fermentation operation provides another offtake avenue that prevents them from having to burn the gas off.
Good chemistry
It’s often said that without a petroleum industry, there will be no chemicals industry. But the chemicals sector, like so many others, is now looking to decouple from fossil fuels. New methods are being developed by companies like DMC Biotechnologies to use microbes to produce an ever-growing catalogue of chemicals and intermediates that can be used across many applications.
Previous attempts at creating chemicals through microbes tended to focus on one or two end compounds. Despite some successful projects in the past, it ultimately took too much time and money to scale them, as each new strain and scale required teams to go back to the process engineering, according to DMC’s co-founder and CEO Matt Lipscomb.

DMC has taken a different approach, says Lipscomb, creating a standardised process to engineer the microbe, thereby eliminating much of the leg work that would otherwise be involved to produce at any scale, making it predictable and somewhat standardised.
What DMC now has is a library of what it calls “chassis strains”, which have been pre-optimised for various combinations that result in different chemicals on the other side, provided that different routes are selected along the organism’s metabolic pathway. Dextrose is the main feedstock used, and when combined with different sets of enzymes and different energy needs, you will end up with different chemicals.
“There may be 16, 20 or 25 different chemical conversion sets that are all happening within the microbe – that’s what all the different enzymes do, and the microbe itself has the native machinery, the chassis, if you will, to get to a lot of that,” says Lipscomb.
He describes what happens inside the cell as a river with different branches. By throwing different enzymes into the mix, you can redirect where the carbon and energy go within that metabolic flowchart and ultimately end up with a different chemical.
“One set of metabolism reaction kind of gets you to alanine or certain other amino acids, another set gets you to the terpenoids family, another set gets you to the fatty acid family.”
DMC’s main offering is alanine, an amino acid that can be used to make a range of pharmaceutical products, health supplements or other products like detergents, but it has several other amino acids in development. These include valine, leucine and isoleucine, which can be used in human and animal nutritional products. Low- calorie sweetener xylitol is also in the pipeline, along with a wide range of compounds that have yet to be announced.
DMC uses E.coli as the primary microbe platform, mainly because it already is the best-understood microbe in the world, and the company is looking at using a yeast host. Between those two, you can cover most of what is possible through microorganisms, according to Lipscomb.
Reaching commercial scale
DMC has started to reach real commercial scale since raising a series A round in 2019, through which it was able to take multiple products to commercial scale. It was able to demonstrate viability at a 3,000-litre scale at a facility in Delft and was able to take its lead product to full 85,000-litre commercial scale in Germany in 2021.
This kind of technology brings the costs down to the point where chemicals are in play, according to Matt Jones, managing director at Solvay Ventures, which backed DMC as part of its $39m series B round last year, and whose parent company is in search of more sustainable chemical inputs and diversifying away from petroleum.
“Historically, it’s always been you can use these biological processes for pharmaceuticals, more and more you can use them for food. Cost and price points and value points to also disrupt chemistry requires this third wave, and so what excites us is that the techno-economics are getting to the point where it can actually work as a displacement of petroleum,” says Jones.
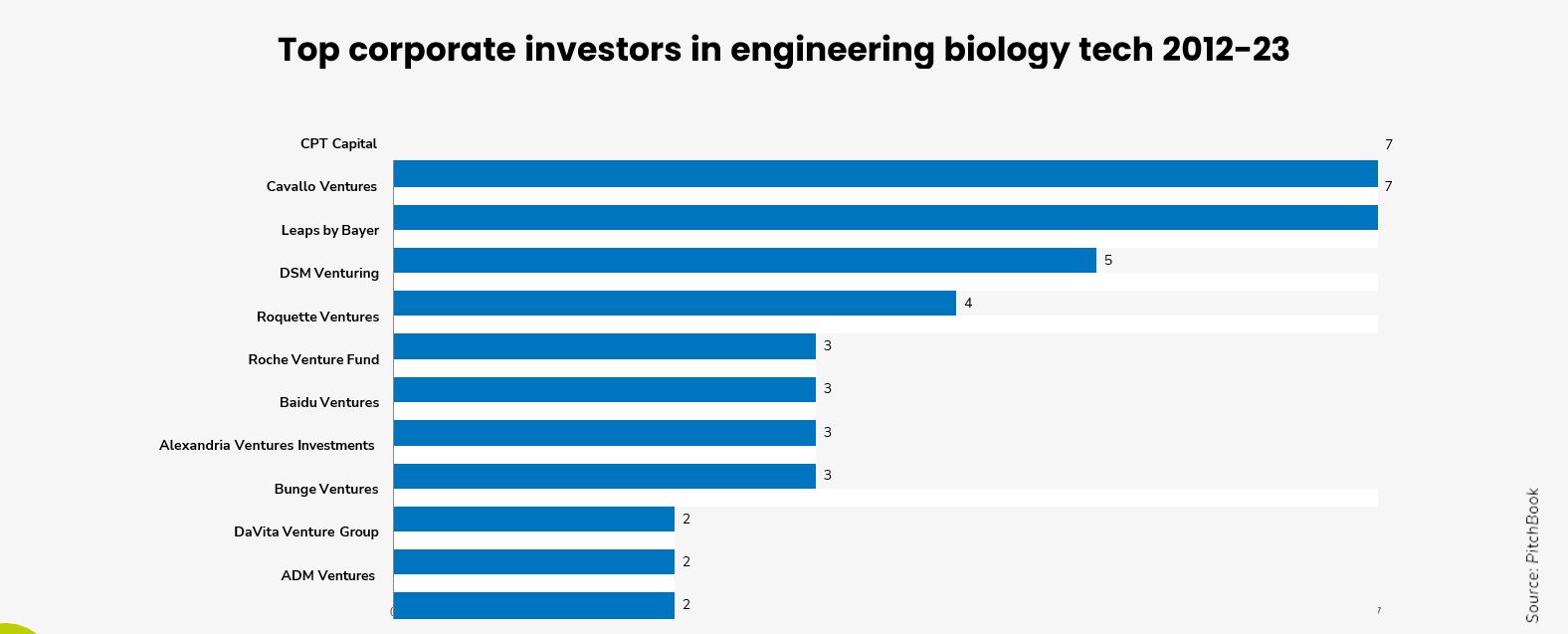
One bright spot is the growing buy-in from corporates – both in terms of investment and off-take – for sustainably-sourced industrial materials compared to 20 years ago.
Coppelia Marincovic, partner at Solvay Ventures, says DMC was one of the few biotech companies it has seen that can actually deliver on cutting costs on chemicals. “If you go into specialities that are very expensive, which a lot of other biotech companies are doing, it’s hundreds of dollars per kilogramme, so you’re tackling really small markets. DMC’s technology enables almost semi-commodity markets – very large markets – and so this is why we think this is transformational.”

Limitations
Plenty of obstacles remain, however, before micro-based chemicals go mainstream. The most obvious one is simple capacity constraints – it takes an eye-watering amount of money to put steel in the ground and get facilities built, which is exacerbated in today’s fundraising environment where both equity and debt are much harder to come by than they were 18-24 months ago.
The economics around biofuels and sustainable aviation fuels are difficult, but not impossible. Feedstock prices for SAF are almost as high as the fuel itself, and given the quantities of fuel the airline industry needs, it will take time for alternative fuels to make a dent in terms of market share.
One bright spot is the growing buy-in from corporates – both in terms of investment and off-take – for sustainably-sourced industrial materials compared to 20 years ago, especially the more customer-facing corporates.
Governments also have their part to play in the push for more sustainability. Legislation such as the Inflation Reduction Act in the US, and its heavy climate focus, also incentivise companies to cut their fossil fuel use.
Finally, there is an inertia factor. A relative lack of success stories in the space hasn’t helped its momentum, according to Lipscomb. “We need more proof points because there is still a fair bit of very valid criticism. If you look at the 2000s and some of the companies that have gotten public in the last couple of years, it’s been a pretty rocky road. The industry needs some successes to really build the credibility and get the flywheel turning.”
